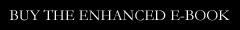UPDATE: So I spent 24 hour feverishly writing another script based on an episode of SModcast – this time SModcast # 52: The (C)Rapture. I got about 20 pages in when I realized there was no way to write this inexpensively as a feature film. Everything was requiring too many effects that I could never achieve using in-camera tricks like forced perspective. It was starting to feel like a pricier project than I know I’d ever be able to find money to make.
And then I remembered this is a SModcast Picture. What do we do on SModcast sometimes? We sing. We make up stupid songs.
And my favorite piece of art ever produced by human hands is the glorious BOOK OF MORMON.
So… I started reshaping the HELENA HANDBAG script as a piece of SMusical THEATER, with BOOK OF MORMON as my spirit animal!
And holy shit… does THIS feel right! After making up all those goofy tunes on EDUMACATION and PLUS ONE, not to mention the FAT MAN ON BATMAN theme song, this feels like a logical progression. And it’ll be fun (and way easier) to stage in a theater! I’m a marginally-straight 43 year old fat man who has always loved show tunes: I guess I’d better finally try writing a musical.
So there you go: another look inside the creative process. One day, you think you’re writing a movie, the next day you realize it’s that tuner you’ve always wanted to write instead. And making up songs? That’s the kind of whimsy I can push forward even if I’m standing in the shower (where I normally only masturbate and try to reach hard-to-wash areas with a loofah on a stick). I thought CLERKS: THE MUSICAL would be my first attempt at theater one day. So happy to realize it’s gonna be something original instead.
And with that, I look to the heavens and shout “FATHER! The fat sleeper who was in the Henry Hudson Regional late-80′s performances of DAMN YANKEES and YOU’RE A GOOD MAN, CHARLIE BROWN, and GREASE has awakened! (Here is proof that theater is in my blood: me as Kenickie in GREASE!)
PREVIOUS ENTRY:
The non-buried lead: I’m writing this new flick based on another episode of SModcast from 2008. Also: I talk about my future in filmmaking. If you feel brevity is the soul of wit, you can stop reading now. If you like to read, here we go…
On the most recent episode of SModcast, I talked about my new mantra in regards to filmmaking: from now until I drop dead, I’m only ever gonna make a flick that only I would/could ever make. Jersey Girl, Zack and Miri Make a Porno, Cop Out – while I love them all, these are movies anybody could make. Whether you like ‘em or hate ‘em, nobody else but me could’ve (or would’ve) ever made Clerks. Or Chasing Amy. Or Dogma. Or Red State. Or Clerks II. Or Mallrats. Or Jay & Silent Bob Strike Back. I let my view askew get standardized for awhile there – so much so that I was happy to walk away from it all for three years and do other shit. But after conceiving and shooting TUSK in less than 6 months (with the help of a shit ton of amazing professionals and a budget smaller than that of RED STATE), I realized that film isn’t in my blood… my films are in my blood. And some of ‘em are still in there – so I guess I’d better get ‘em out.
Now that I’ve spent the last three years clothing/feeding/housing me and my family without a dime of movie money, I know that I don’t need film to pay my bills anymore. So I can make a movie when I feel like it – and if I’ve got nothing to say in a screenplay, I can just fuck off for awhile and do other shit until I do have a movie in me. Like I think I do right now.
January will conclude with me and my Tusketeers getting together again – this time in Los Angeles, to shoot all the Guy Lapointe scenes we still owe for Tusk. Our composer Chris Drake (the genius behind The Dark Knight Returns animated feature score) is currently finding the music of Tusk. That means there’s not much for me to do until the end of the month. I’ll be recording podcasts and shooting Spoilers every week (see it in Canada on Comedy every Friday at 9:30; U.S. info coming in January), but when I’m not doing that, I’ve decided to push a little whimsy again – like I did with #WalrusYes. The idea is to, once again, take an episode of SModcast and turn it into a movie. It’s worked out quite nicely with Tusk (thanks largely to the cast, the crew and the good folks at Demarest and at a24, who’ll be releasing the flick this fall), so I wanna see if I can do it again.
Granted, this time the plot of the movie concerns mankind teaming up with Hell to save existence from extinction at the hands of a Rapturing giant Jesus – which means the budget has to be low, because nobody’s gonna wanna make that movie. At all. And I know this going in, so I won’t be heartbroken if it never goes beyond the script. But… if the script is funny enough? Who knows? That walrus movie seemed pretty daffy and far-fetched until we were standing on the Tusk set bringing that shit to life four months and change after first making it up in episode 259 of SModcast. Right now, the aim is to shoot Clerks III this May, and then there’s Hit Somebody after that – so this Helena Handbag picture would still be a ways off anyway. But why sit around commenting on other people’s lives and art on the internet when I can try to make some new art instead?
As for the title: If you heard the podcast, you know Scott suggested Christzilla in place of Holy Christ! – the title of the fake movie in the original podcast. I asked the audience to offer up their suggestions at #BeatChristzilla and they were all really fun! But I’m going with the title Helena Handbag – which comes directly from the plot of the flick (it’s kind of a nod to David Lynch’s unproduced One Saliva Bubble screenplay, too – which was also about the end of the world and also carried a title that didn’t really prepare you for what the movie was about). So I’m off to write Helena Handbag.
I’m gonna end this by urging you all to do something similar to what I’m doing: put a whimsy on wheels today. No pressure and it doesn’t have to result in anything: it is, after all, just a whimsy. Tend to it in your spare time but really give it care and nurture that fucker – just in case it actually turns into something. And it can turn into something, no matter who you are or how much money you have: I burned my film career to the ground, went and did other shit for three years (mostly for free), started from scratch again from a salted earth – and (unless I somehow fuck up the forthcoming Lapointe scenes) I wound up making the best flick I’ve ever made with Tusk. So go give it a shot, kids: push a little whimsy yourselves. Unless that whimsy involves hurting people. Never kill anything unless it’s trying to kill you.
Today, you can waste lots of time posting your thoughts about what other people are doing/saying in life – or you can use that time more wisely, to create something from nothing that’s all yours. One path is easier, but the other path is way more fun…